Management of Bronchiolitis, what does the evidence say?
- Published on:
Management of Bronchiolitis, what does the evidence say?
- Published on:


Bronchiolitis is a common chest infection that affects babies and children under two and due to its high frequency, acute bronchiolitis ranks among the costliest children’s conditions in western countries [1].
Common early symptoms seen are like a cold, such as sneezing, a runny or blocked nose, a cough and a slightly high temperature of 38oC [2]. Bronchiolitis is typically self-limiting, mild and can be treated at home. However, some children will need hospital admission due to symptom severity peaking between days 3 and 5 of illness causing persistently increased respiratory effort (eg, flaring, grunting, severe retractions) and hypoxemia [3].
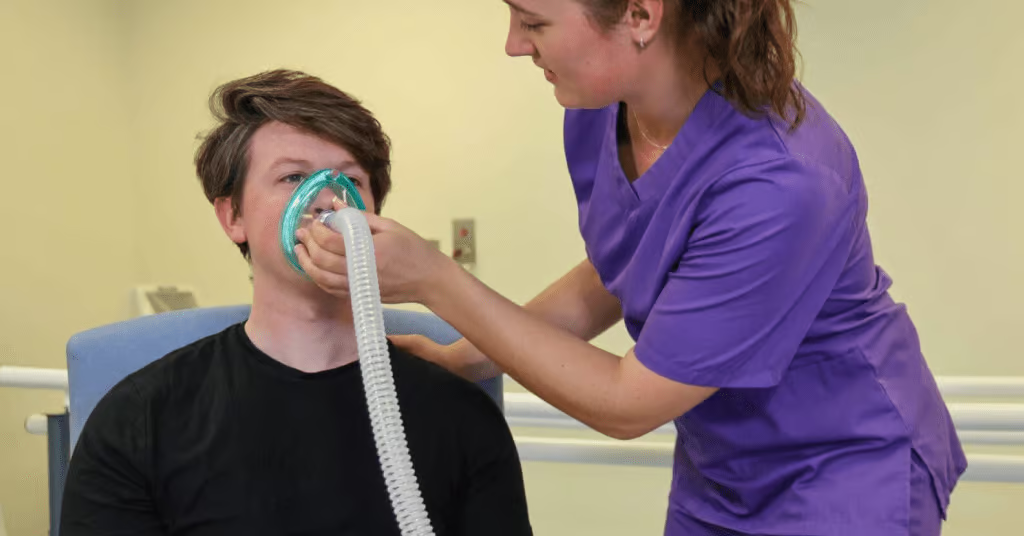
When infants are admitted to hospital, the management is supportive and monitoring for worsening disease: oxygen supplementation, high flow oxygen and treatment of dehydration [4]. The current, evidence-based practice guidelines recommend that infant bronchiolitis should not be routinely treated with any medication as it is mostly viral [4, 6].
Besides, unnecessary administration of medication has been proven to have low clinical effectiveness in the treatment of bronchiolitis and can pose potential serious side effects, therefore it is vital for successful treatment that clinicians practice in accordance with evidence-based practice recommendations and guidance.
From clinical observation the author has seen the rapid rise in utilising HHFOT (Humidified High Flow Oxygen Therapy) within the ward environment in the management of bronchiolitis and early initiation may be important for success of treatment option. Moreover, treatment option has shown promising results in the reduction of respiratory distress in acute viral bronchiolitis where evidence also suggested that in infants with viral bronchiolitis and moderate-severe respiratory distress, HHFOT is a beneficial therapy which can be safely used outside the intensive care area [5] as the therapy reduces the work of breathing, improves oxygenation, and may reduce need for intubation.
Even though historically non-invasive ventilation may have reduced intubation rates and potentially health care costs the author feels that this needs to be translated into research as it is currently lack of robust studies to confirm this.
References
ARDS Definition Task Force, Ranieri, V. M., Rubenfeld, G. D., Thompson, B. T., Ferguson, N. D., Caldwell, E., Fan, E., Camporota, L., & Slutsky, A. S. (2012). Acute Respiratory Distress Syndrome. JAMA, 307(23), 2526–2533. https://doi.org/10.1001/jama.2012.5669
Gattinoni, L., Taccone, P., Carlesso, E., & Marini, J. J. (n.d.). Concise Clinical Review Prone Position in Acute Respiratory Distress Syndrome Rationale, Indications, and Limits. https://doi.org/10.1164/rccm.201308-1532CI
Griffiths, M., & Baudouin, S. (2018). GUIDELINES ON THE MANAGEMENT OF ACUTE RESPIRATORY DISTRESS SYNDROME. https://www.ficm.ac.uk/sites/default/files/ficm_ics_ards_guideline_-_july_2018.pdf
Guérin, C., Reignier, J., Richard, J.-C., Beuret, P., Gacouin, A., Boulain, T., Mercier, E., Badet, M., Mercat, A., Baudin, O., Clavel, M., Chatellier, D., Jaber, S., Rosselli, S., Mancebo, J., Sirodot, M., Hilbert, G., Bengler, C., Richecoeur, J., … Ayzac, L. (2013). Prone positioning in severe acute respiratory distress syndrome. The New England Journal of Medicine, 368(23), 2159–2168. https://doi.org/10.1056/NEJMoa1214103
Tanaka, L. M. S., Azevedo, L. C. P., Park, M., Schettino, G., Nassar, A. P., Réa-Neto, A., Tannous, L., de Souza-Dantas, V. C., Torelly, A., Lisboa, T., Piras, C., Carvalho, F. B., de Oliveira Maia, M., Giannini, F. P., Machado, F. R., Dal-Pizzol, F., de Carvalho, A. G. R., Dos Santos, R. B., Tierno, P. F. G. M. M., … Salluh, J. I. F. (2014). Early sedation and clinical outcomes of mechanically ventilated patients: a prospective multicenter cohort study. Critical Care (London, England), 18(4), R156. https://doi.org/10.1186/cc13995

Kaltoon Mohamed Ali
Professional Development Nurse