One or two pressures: The differences between CPAP and Bilevel PAP
- Published on:
One or two pressures: The differences between CPAP and Bilevel PAP
- Published on:

On this page

Healthcare companies use various brand names or anacronyms labelled for similar treatments. We all know what treatment is best for our patient, but it quite often depends on what available equipment you have within your reach in your hospital.
Care aimed at treating respiratory failure varies between patient illness and patient types and we find care can also differ between hospitals. Choosing which treatment to give often depends on local protocols and these can vary between hospitals.
Within the UK, we tend to use the BTS (British Thoracic Society) [1] and NICE (National Institute for Health and Care Excellence) [2] guidelines as a baseline to create protocols for administering NIV (non-invasive ventilation) and CPAP (continuous positive airway pressure). NICE provides national guidance and advice to improve health and social care whereas the BTS provide guidance and suggest quality standards with respiratory illnesses and treatments. There are other guidelines that are used in different areas, but we will concentrate on these for now.
NIV and CPAP procedures are particularly favoured within respiratory healthcare, perhaps because the treatments can be used in multiple areas throughout the hospital, therefore needing an overarching care pathway and strict protocols. NICE discuss the importance of many factors relating to combating side effects of NIV, such as developing and implementing a patient-centred NIV care bundle and algorithm in line with NICE, BTS and by referring to the NCEPOD (National Confidential Enquiry into Patient and Outcomes and Deaths) [3] for setting standards and recommendations.
European countries differ in their treatments too but there are recommendations discussed by the ERS (European Respiratory Society) that were last published in 2017 stating where bilevel or CPAP should be used for which patient group. [4]
So, what is the difference between the two treatments?
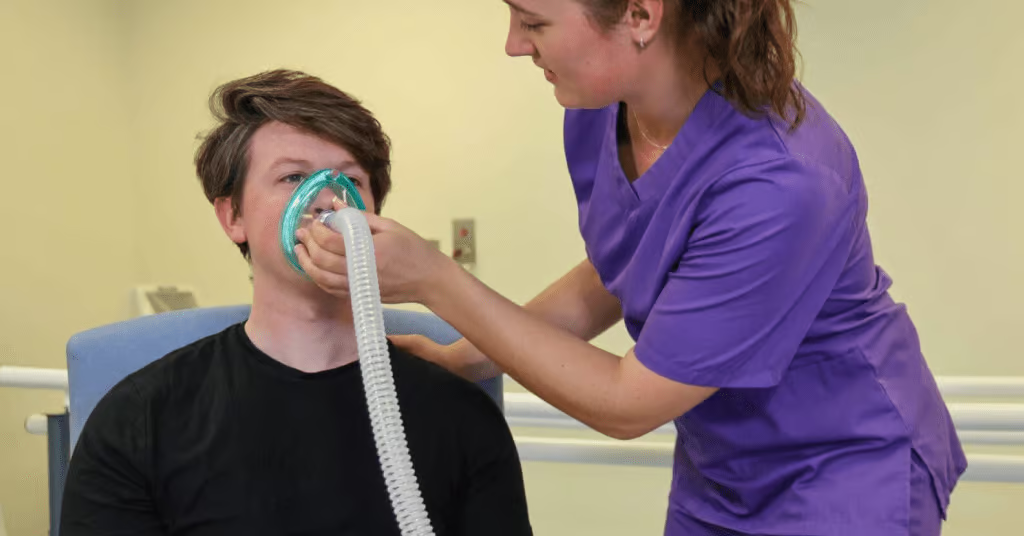
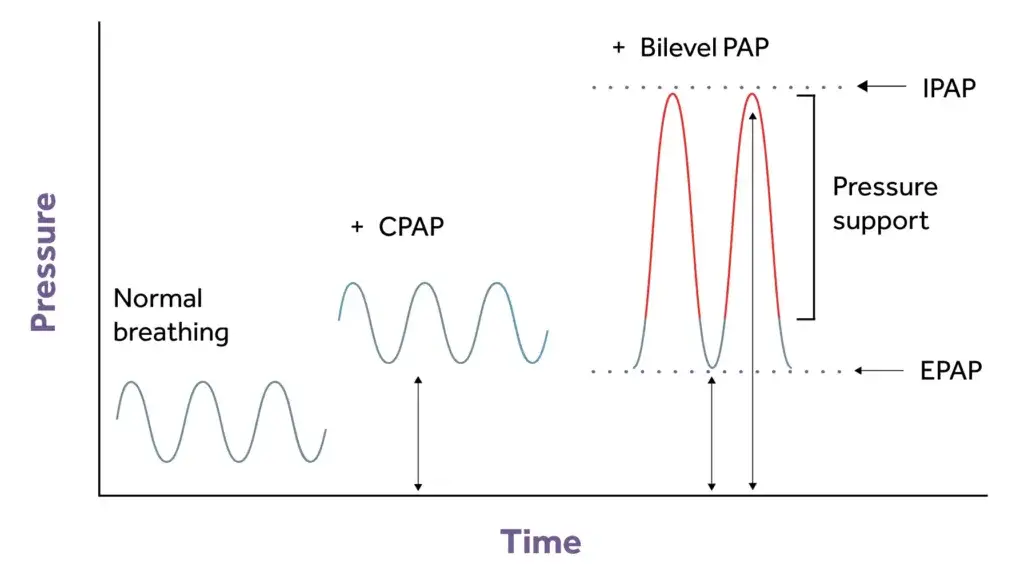
CPAP is a non-invasive respiratory treatment with a mask, a tracheostomy or a helmet that treats a patient’s hypoxaemia by using a PEEP valve or a PEEP starting setting of 5cm H2O. As the name suggests, CPAP is a continuous PAP (positive airway pressure) throughout the whole cycle of the patients’ breath and the patient is required to be spontaneously breathing throughout. CPAP allows the airways to remain splinted open to allow gaseous exchange to take place and aids recruitment of alveoli in a patient with atelectasis, therefore increasing oxygen saturation levels.
Bilevel is a term used to describe bilevel positive airway pressure. Other manufacturers may use the terms NIPPV, bilevel PAP and S/T (spontaneous/timed) and often NIV. Bilevel may help those who are struggling to acclimate to CPAP and is generally used to treat hypercapnia, a low pH (7.25-7.35 mmHg) and acidosis. It is a non-invasive respiratory treatment using a mask or a tracheostomy. It may improve air swallowing (called aerophagia) and may also help with claustrophobia. Bilevel may be required when pressures are higher to improve comfort, especially at positive airway pressures that are 15 cm H2O pressure or higher. The “bilevel” component refers to the fact that there are two pressures, which the machine can alternate between. This allows you to breathe in with higher pressure and breathe out against a slightly lower pressure [5].
Unlike CPAP, where usually no back-up breaths are provided, if a patient were to become apnoeic with bilevel, the device provides a back-up breath preset by the healthcare professional.
What to use and when?
NICE and the ERS suggest the following rationale for using bilevel; to treat persistent hypercapnic ventilatory failure and acidosis during an exacerbation of COPD, when a person’s arterial blood gases (especially the pH and carbon dioxide levels) are not responding (or worsening) despite optimal medical management [4].
In the BTS guidelines, the referral to the use of CPAP is that it improves oxygenation in patients with diffuse pneumonia who remain hypoxic despite maximum medical treatment and CPAP has been shown to be effective in patients with cardiogenic pulmonary oedema who remain hypoxic despite maximal medical treatment [1].
NICE discuss that non-invasive ventilation should be delivered in a dedicated setting by staff trained and experienced in its use [6] and this is a vital discussion when it comes to device allocation, staff skill and best practice for patient safety for optimal treatment throughout the hospital. Patients’ needs must be closely monitored whilst receiving treatment and an escalation treatment plan needs to be in place before commencing therapy.
It is important to understand the differences between CPAP and Bilevel PAP as they are different positive pressure treatments and choosing which to deliver will be based on patient assessment.
References
Crimi, C. et al (2022). High-flow nasal oxygen versus conventional oxygen therapy in patients with COVID-19 pneumonia and mild hypoxaemia: a randomised controlled trial. Thorax, 78. https://doi.org/10.1136/thoraxjnl-2022-218806
Frat, J.-P. et al (2015). High-Flow Oxygen through Nasal Cannula in Acute Hypoxemic Respiratory Failure. New England Journal of Medicine, 372(23), 2185–2196. https://doi.org/10.1056/nejmoa1503326
Lim, W. S., Smith, D. L., Wise, M. P., & Welham, S. A. (2015). British Thoracic Society community acquired pneumonia guideline and the NICE pneumonia guideline: how they fit together. Thorax, 70(7), 698–700. https://doi.org/10.1136/thoraxjnl-2015-206881
NHS England. (2022). RightCare Community- acquired Pneumonia Toolkit. https://www.england.nhs.uk/rightcare/wp-content/uploads/sites/40/2022/09/RightCare-Pneumonia-toolkit.pdf
NHS Inform. (2022, December 7). Pneumonia. www.nhsinform.scot. https://www.nhsinform.scot/illnesses-and-conditions/lungs-and-airways/pneumonia/
NICE. (2014, December 3). Introduction | Pneumonia in adults: diagnosis and management | Guidance | NICE. Nice.org.uk; NICE. https://www.nice.org.uk/guidance/CG191/chapter/introduction
NICE guideline. (2023, October 31). Recommendations | Suspected acute respiratory infection in over 16s: assessment at first presentation and initial management | Guidance | NICE. Www.nice.org.uk. https://www.nice.org.uk/guidance/ng237/chapter/Recommendations