- INSPIRE White Paper
NeoFlow® VT Ventilator Circuit With Helical Wire: Performance Evaluation in the Turkish Market
- Product Featured: NeoFlow®

Introduction
This paper will discuss the evaluation of the performance of the Eakin Healthcare NeoFlow® VT ventilator circuit AMVC1775-203 with the new helical wire component AMCA13-006.
Scope
It relates to responses of a commercial product evaluation for the performance of AMVC1775-203 which was conducted in August/September 2025 in Turkey.
Background
During 2024, market feedback was received from Turkish hospitals which were experiencing issues of condensation in the inspiratory limb of NeoFlow® VT ventilator circuit AMVC1775-204. Competitor circuits were being used as an alternative and were remaining dry. A market visit was conducted. It was confirmed that competitor circuits were gathering less condensation than Eakin Healthcare circuits and that hospital staff were moving away from the AMVC1775-204 circuit.
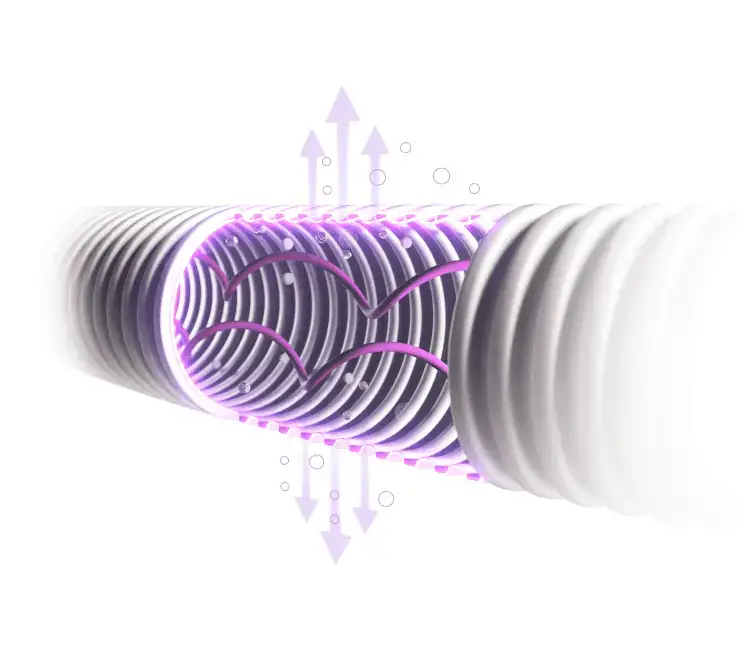
Subsequently, it was identified that the heated inspiratory limb needed improved performance to prevent excessive water condensate from forming inside the tubing during continuous clinical use, for up to 7 days, in both ventilated and continuous flow therapies at ambient room temperatures of 21–25°C.
The decision was made to develop an existing manufacturing process for helical form heater wires. A change was raised to convert the first of the NeoFlow® VT ventilator circuit product codes across from the existing neonatal rectilinear heater wire to the new helical heater wire within the inspiratory limb of the NeoFlow® VT ventilator circuits AMVC1775-203 and AMVC1775-204.
A commercial product evaluation was conducted to gather feedback on the overall performance of AMVC1775-203 in the Turkish market as shown in Image 1.
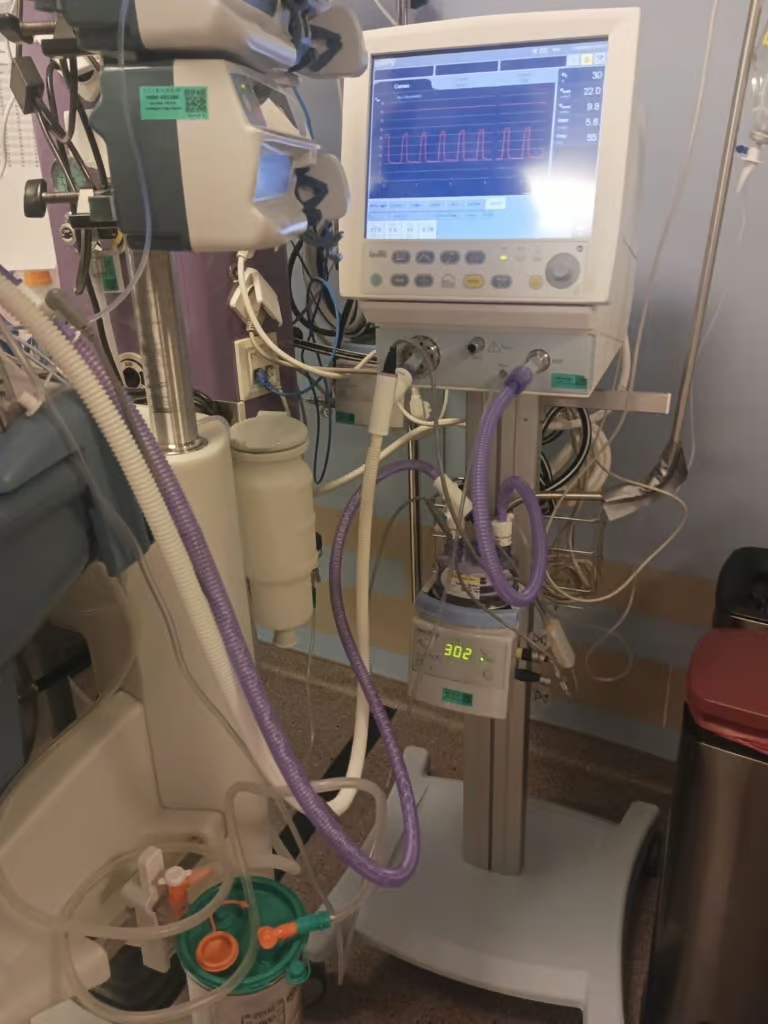
(Image 1: Eakin Healthcare NeoFlow® VT ventilator circuit AMVC1775-203 being evaluated in a Turkish hospital)
Although the circuit used within the Turkish hospitals was AMVC1775-204, this circuit was originally the stop-gap solution for AMVC1775-203. As a result, AMVC1775-203 was chosen for the evaluation. This product evaluation was conducted by Turkish users during August and September 2025 to assess the functioning and water condensate formation of the new inspiratory limb during continuous clinical use.
Objectives
Primary objectives
- Identify the level of water condensate accumulation within the NeoFlow® VT inspiratory tubing across 7-day single patient use.
- Identify the length of time until water condensate accumulated within the NeoFlow® VT inspiratory tubing across 7-day single patient use and if applicable.
- Gather information on the cot location, room temperature and incubator temperature throughout NeoFlow® VT circuit use.
- Assess whether the level of water condensate accumulation was acceptable to continue NeoFlow® VT circuit use if applicable.
Secondary objectives
- Evaluate the ease with which a user can set up the NeoFlow® VT circuit.
- Compare performance of the NeoFlow® VT circuit to competitor ventilator circuits currently used on the ward.
- Gather general product feedback.
Participants
AMVC1775–203 NeoFlow® VT circuit with heated inspiratory limb was planned to be evaluated in the Turkish market on multiple neonatal patients within a selection of different hospitals, all located within Istanbul.
It was confirmed that 5 hospitals were suitable to carry out the product evaluation. The hospitals chosen included those which were previously experiencing condensation accumulation in the inspiratory limb of NeoFlow® VT AMVC1775-204 circuit. The aim was to gather a minimum of 10 evaluation responses.
The product evaluation was conducted at the following hospitals:
- Acıbadem Atakent University Hospital.
- Şişli Hamidiye Etfal Training and Research Hospital.
- Süleymaniye Maternity and Children’s Diseases Training and Research Hospital.
- Zeynep Kamil Women’s and Children’s Diseases Training and Research Hospital.
- Kanuni Sultan Süleyman Training and Research Hospital.
Data Collection
Evaluation data was collected via a commercial product evaluation form, available in either English or Turkish. The evaluation form was provided in both hard copy and as an online version.
Hospital staff were asked to collate the following data during the product evaluations:
- Start and end date of NeoFlow® VT circuit usage on each patient.
- Location of the cot within the neonatal intensive care unit (NICU).
- Temperature of the incubator whilst the NeoFlow® VT circuit was being used at both the start and end of the therapy.
- Temperature of the room whilst the NeoFlow® VT circuit was being used at both the start and end of the therapy.
- Level of water condensate accumulation within the NeoFlow® VT inspiratory tubing (if applicable).
- Acceptability of water condensate accumulation within the NeoFlow® VT inspiratory tubing (if applicable).
- Company and brand name of competitor circuitry currently used.
- Performance of the NeoFlow® VT circuit compared to competitor circuitry currently used.
- General product feedback.
All data received was consolidated and analysed.
Results
The following results analyse and report on the 10 evaluation responses received.
Patient Hospital Locations
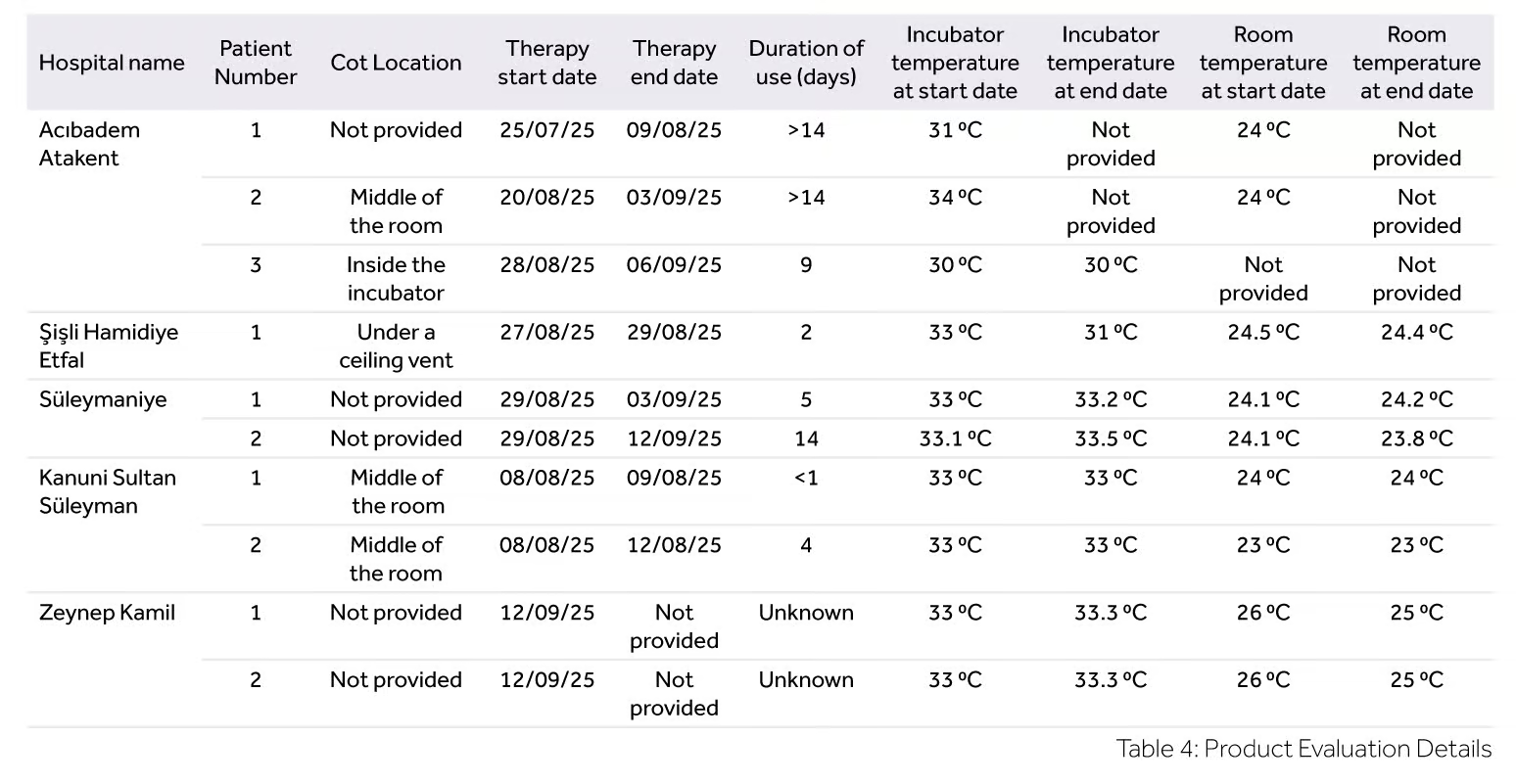
The 10 evaluation responses were received from the hospitals detailed in Table 3.
Evaluation Environment Details
The cot location was not provided for 50% (n=5) of the patients, 30% (n=3) of the cot beds were located in the middle of the room and 10% (n=1) of the cot beds were located under a ceiling vent. One patient location of the cot bed is recorded as in the incubator.
At the start of therapy, the room temperature was recorded within the range of 23-26°C for 90% (n=9) of the patients. For 10% (n=1) of the patients this information was not provided. At the end of therapy, the room temperature was recorded within the range of 23-25°C for 80% (n=8) of the participants. For 20% (n=2) of the patients this information was not provided.
Although longer than the recommended usage duration of 7 days, the NeoFlow® VT circuit was used on 40% (n=4) of patients for 9 – 14 days. All evaluation location and temperature details are summarised in Table 4.
Product Observations
Respondents were asked to provide feedback on water accumulation, competitor circuitry and general product feedback. Results from the respondents for each question are discussed in this paper.
Circuitry Set Up
In relation to the first question: “Was the circuit easy to set up on the patient?”, 100% (n=10) of the respondents selected “Yes”.
Water Condensate Accumulation
The second question asked: “Whilst using this NeoFlow® VT circuit did water condensation build up within the tubing?”, 90% (n=9) of the respondents selected “No”. Water condensation build up within the tubing was observed on one circuit (10%) during the evaluation on a patient within the Kanuni Sultan Süleyman Training and Research Hospital.

Upon answering yes to water condensate accumulation, further questions were asked of the respondent to determine the amount of water accumulation. They indicated that a small amount of water was observed at the lowest point of the tubing during “half a day – 1 day” after therapy had started.
The same respondent was further asked: “Is the level of water build up acceptable, or would it stop you from using the product?”. They selected “No”. However, on review of this sub section of question 2, it has been noted that this question was ambiguous. As the therapy end date was one day after therapy initiation, it can be surmised that, in this instance, the respondent was answering “No” to the first part of the question: “Is the level of water build up acceptable?”.
Additionally, the one respondent within the Şişli Hamidiye Etfal Training and Research Hospital selected “No” to water condensation build up within the tubing but proceeded to answer some of the sub sections of question 2, which were only intended to be answered if they answered “Yes” to water accumulation. They indicated that the level of water accumulation was “Droplets forming on the tubing walls but not a significant amount of water observed gathering in the lowest point of the tube”. In this instance, it cannot be surmised what the respondent intended with the ambiguous question “is the level of water build up acceptable, or would it stop you from using the product?”, as the circuit was only used for 3 days, yet they also indicated that the circuit was performing the same as their current Fisher & Paykel Healthcare RT265.
Competitor Circuitry Currently Used
Within the 5 Turkish hospitals in which the product was evaluated, 40% (n=4) of the respondents currently use Fisher & Paykel Healthcare circuits, 20% (n=2) currently use a combination of both Fisher & Paykel Healthcare and Flexicare circuits, 20% (n=2) currently use a combination of both Fisher & Paykel Healthcare and Hudson circuits and 10% (n=1) use Hudson circuits only. One respondent within the Kanuni Sultan Süleyman Training and Research Hospital did not provide any information.
Two respondents stated that they currently use Fisher & Paykel Healthcare RT265. One respondent confirmed that they currently use the Hudson 780-14 circuit.
Competitor Circuitry Comparison
o get a comparison of our NeoFlow® VT circuit performance versus our competitors, question 5 asked, “Is this NeoFlow® VT circuit performing better than the circuit you currently use on the ward?”. Only 30% (n=3) of respondents selected a direct “Yes” or “No” answer on the evaluation form. From these, one respondent from Süleymaniye Children’s Hospital selected “Yes”, the NeoFlow® VT circuit was performing better than their current Hudson circuit. One respondent from Şişli Hamidiye Etfal Training and Research Hospital, selected “No” to this question but also commented that it performed the “same” as their current Fisher & Paykel Healthcare RT265 circuit.
As identified in question 2, water condensation build up within the tubing was observed during an evaluation on one patient within the Kanuni Sultan Süleyman Training and Research Hospital. The same respondent indicated that the NeoFlow® VT circuit did not perform better than the Fisher & Paykel Healthcare RT265 and Hudson 780-14 circuits which they currently use on their ward. They commented, “HFO- there is too much water and when you try to change the mode the device is not working.” As this was an evaluation in a hospital environment we were unable to investigate this any further.
The remaining 70% (n=7) of respondents did not select either a “Yes” or “No” answer to this question. However, 3 of these respondents provided some additional comments explaining the product was being trialled. One respondent from the Süleymaniye Children’s Hospital provided an additional comment explaining, “Our circuit is better than Hudson and same performance as Fisher and Paykel.”
Conclusion
Primary objectives
- Identify the level of water condensate accumulation within the NeoFlow® VT inspiratory tubing across 7-day single patient use.
- Identify the length of time until water condensate accumulation within the NeoFlow® VT inspiratory tubing across 7-day single patient use.
- Gather information on the cot location and temperature throughout NeoFlow® VT circuit use.
- Assess whether the level of water condensate accumulation was acceptable to continue NeoFlow® VT circuit use.
Overall, based on the responses to date (n=10), NeoFlow® VT ventilator circuit AMVC1775-203 was deemed to be acceptable in relation to 9 users within the following 5 Turkish hospitals:
- Acıbadem Atakent University Hospital.
- Şişli Etfal Training and Research Hospital.
- Süleymaniye Maternity and Children’s Diseases Training and Research Hospital.
- Zeynep Kamil Women’s and Children’s Diseases Training and Research Hospital.
- Kanuni Sultan Süleyman Training and Research Hospital.
Therefore, a pass criterion of 90% was achieved for the key performance variable:
- Assess whether the level of water condensate accumulation was acceptable to continue NeoFlow® VT circuit use.
Secondary objectives
- Evaluate the ease with which a user can set up the NeoFlow® VT circuit.
- Compare performance of the NeoFlow® VT circuit to competitor ventilator circuits currently used on the ward.
- Gather general product feedback.
Overall, based on the responses to date 100% (n=10) of all evaluation respondents were satisfied with the ease of use in setting up the NeoFlow® VT circuit. Two respondents indicated that the NeoFlow® VT circuit performed the same as Fisher & Paykel Healthcare RT265 circuit.
Overall findings to date demonstrate that the introduction of the helical wire component AMCA13-006 in NeoFlow® VT ventilator circuit AMVC1775-203 has improved its performance by preventing excessive water condensate from forming inside the tubing during continuous clinical use, for up to 7 days, in both ventilated and continuous flow therapies at ambient room temperatures of 21–25°C.

Profile
Lindsay McQuillan
Senior Product Manager
Roanne Lecky
Product Management Assistant